Designing molecular switches using AI
Researchers have succeeded in synthesizing new molecules that mimic the complex behavior of certain biological molecules using AI algorithms inspired by statistical physics.
References :
Designing molecular RNA switches with Restricted Boltzmann machines, Jorge Fernandez-de-Cossio-Diaz, Pierre Hardouin, Francois-Xavier Lyonnet du Moutier, Andrea Di Gioacchino, Bertrand Marchand, Yann Ponty, Bruno Sargueil, Rémi Monasson, Simona Cocco, Nature Communications 16, 11223 - Published: 18 December 2025.
DOI: 10.1038/s41467-025-66265-y (available in open access)
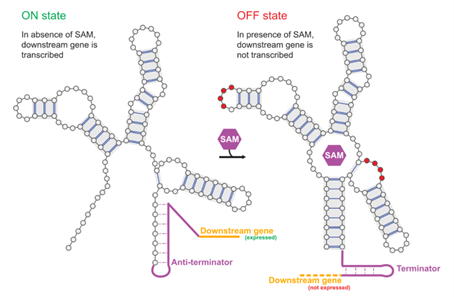
In biology, many RNA molecules act as sophisticated microscopic machines. Among them, riboswitches function as tiny biological sensors, changing their three-dimensional shape when they bind to a specific metabolite. This change in shape often acts as a switch, activating or deactivating a downstream gene. The ability to design these artificial switches from scratch would therefore offer interesting prospects for synthetic biology, drug design, and new diagnostic tools. However, designing a sequence capable of folding stably into two different shapes and switching between them is an extremely difficult challenge.
The present study was carried out in the following CNRS laboratories:
Laboratoire de Physique de l’Ecole Normale Supérieure de Paris (LPENS, CNRS / ENS-PSL / Sorbonne Université / Université Paris Cité)
Institut de physique théorique (IPhT, CEA/CNRS)
Cibles Thérapeutiques et Conception de Médicaments (CiTCoM, CNRS/Université Paris Cité) - CNRS Chemistry
Laboratoire d'Informatique de l'Ecole Polytechnique (LIX, CNRS/Ecole Polytechnique) - CNRS Informatics
A multidisciplinary team of researchers has used machine learning methods to design entirely new functional RNA switches. The researchers applied a model known as “restricted Boltzmann machines” (RBMs) to learn the design rules of the aptamer domain (the part of the riboswitch responsible for recognizing and binding a specific metabolite) of a family of natural riboswitches. By training the RBMs on thousands of natural sequences from various organisms, the model learned to capture the complex correlations characterizing these sequences, due in particular to secondary and tertiary contacts (the name given to specific interactions between distant sites along the sequence, which are close together in space when the RNA molecule is folded), which simpler models were unable to take into account.
The trained RBM was then used as a “generator” to create 476 new RNA sequences, some of which differed by up to 40% from known natural sequences. The team synthesized and tested these artificial sequences using high-throughput chemical probes. The results were remarkable, as, like their natural counterparts, about one-third of the top-scoring sequences designed functioned as effective switches, changing their conformation in response to the target metabolite (SAM).
This work represents a significant advance in the rational design of allosteric RNA molecules, which are capable of adapting to their biochemical environment by changing shape. It demonstrates how generative models, born from the intersection of statistical physics and AI, can decipher the complex “code” of biomolecules that links their sequence and shape, paving the way for tailor-made molecular tools. These results are published in the journal Nature Communications.