Unexpected structural complexity at the heart of superionic water
Researchers have uncovered unexpected structural richness in the solid phases of superionic water, a state achieved under extreme pressures found at the core of planets.
References :
Andriambariarijaona, L., Stevenson, M.G., Bethkenhagen, M. et al., Observation of a mixed close-packed structure in superionic water, Nature Communications - Published: 7 December 2025.
DOI : 10.1038/s41467-025-67063-2
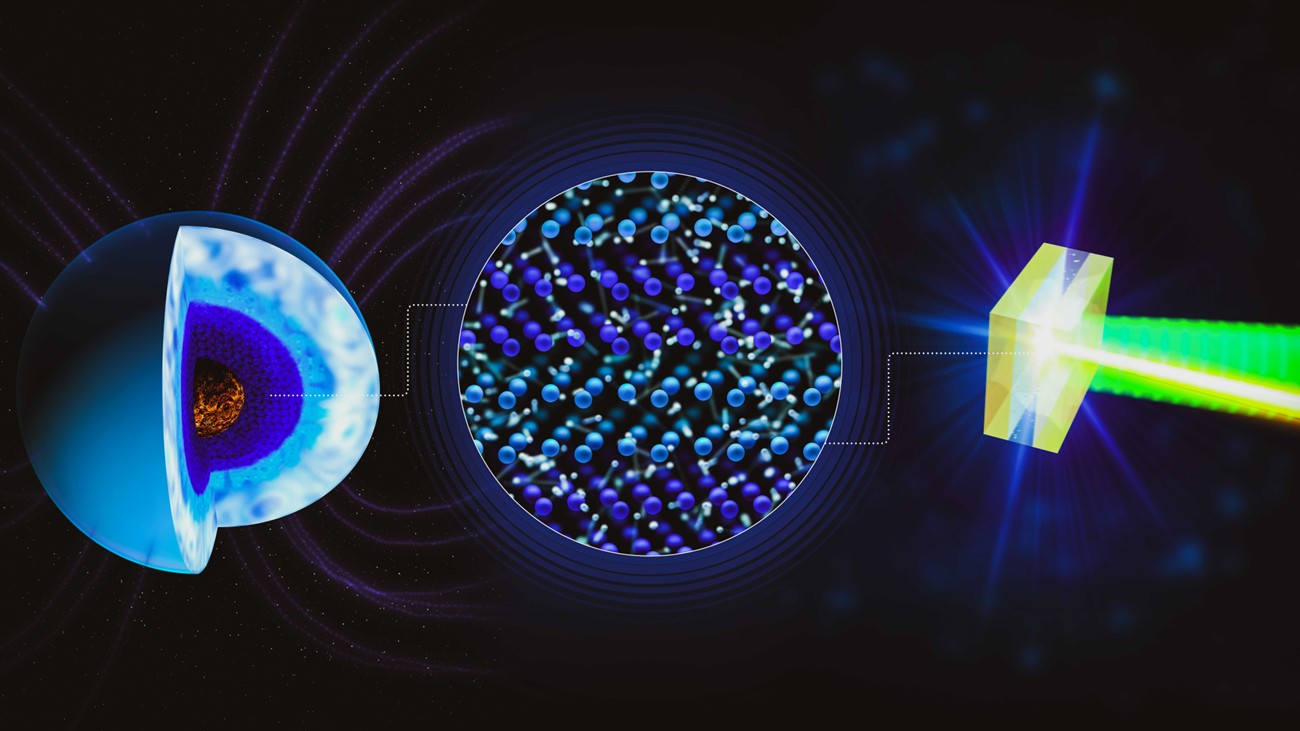
Under extreme pressure and temperature conditions, water adopts a unique state, somewhere between solid and liquid: the superionic phase. This phase is characterised by a crystalline lattice of oxygen atoms, similar to that of a solid, within which hydrogen atoms, in the form of ions, move freely, as in a liquid. Although this phase has already been observed experimentally, many questions remain about its melting curve and the stability of the different oxygen networks that can form within it. These issues are essential to understanding the mechanical and transport properties of this atypical ice, as well as its potential role in generating the magnetic fields of Uranus and Neptune.
The present study was carried out in the following CNRS laboratory:
- Laboratoire pour l'utilisation des lasers intenses (LULI, CNRS / École Polytechnique / Sorbonne Université)
- Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie (IMPMC, CNRS / Museum National d'Histoire Naturelle / Sorbonne Université)
A team of researchers from a large international collaboration, has just taken a major step forward in the exploration of these extreme phases. During two experimental campaigns conducted at the Linac Coherent Light Source (LCLS, Stanford) and the European free-electron laser (XFEL, Hamburg), the researchers compressed a thin layer of water to pressures millions of times higher than atmospheric pressure, while reaching temperatures of several thousand degrees, an experimental domain that had previously been very difficult to access. During this compression, a beam of ultra-fast X-rays (on the order of a few tens of femtoseconds) passed through the sample. Analysis of the diffraction patterns obtained then provides precise information on the organisation of the oxygen atoms. Previous studies suggested that, in superionic ice, these atoms are organised in a body-centred cubic (BCC) or face-centred cubic (FCC) lattice, two common variants of cubic crystal structures. However, the new study reveals a much more complex reality. The researchers show that superionic water adopts a hybrid structure, combining face-centred cubic and hexagonal close-packed (HCP) stacking. The latter corresponds to a stacking of densely packed layers of atoms in a hexagonal geometry. The coexistence of these two patterns creates numerous stacking defects: rather than organising themselves into a single regular configuration, the oxygen atoms form a hybrid sequence, which could only be detected thanks to high-precision measurements using latest-generation X-ray lasers.
In line with predictions from advanced ab initio calculations, these observations show that the structures adopted by water in its superionic state are much more complex than expected. They demonstrate that the superionic regime has a structural richness comparable to the multiplicity of solid ice phases, confirming that water, despite its apparent simplicity, continues to surprise us with physical behaviours that are as remarkable as they are unexpected. This complexity could contribute to shape the macroscopic properties of superionic water, with notable consequences for our understanding of the internal properties of planets such as Uranus and Neptune, and even other extrasolar planets of the same type. These results are published in the journal Nature Communications.