When it comes to cutting a molecule, use an insulated work surface!
In a recent study, researchers have shown that transferring the concepts of synthetic chemistry in solution to a surface can achieve exceptional reaction selectivity, provided that the process is carried out in the vicinity of an insulating surface.
References :
Mélissa Hankache, Valentin Magné, Elie Geagea, Pablo Simón Marqués, Sylvain Clair, Luca Giovanelli, Christian Loppacher, Emmanuel Fodeke, Sonia Mallet-Ladeira, Eddy Maerten, Claire Kammerer, David Madec, Laurent Nony, Photoinduced Modulation of the Oxidation State of Dibenzothiophene S-Oxide Molecules on an Insulating Substrate, Nature Communications 16, 4841 – Published: 24 May 2025.
Doi : 10.1038/s41467-025-60075-y
Open access archives : HAL
Surface chemistry aims to overcome the limitations of conventional solution synthesis by exploiting two-dimensional confinement to create ordered molecular structures with well-controlled properties. To date, most of the work reported in the literature on this ‘interface chemistry’ has been carried out on metal substrates and is based on unconventional reaction mechanisms, a specificity that prevents direct transposition to well-established organic reaction surfaces in solution. Furthermore, as the intrinsic properties and reactivity of metal substrates often limit the activation of surface reactions, photoinduced processes are particularly difficult to exploit on these surfaces due to the optical inhibition of adsorbed molecules that they induce.
The present research was conducted in the following CNRS laboratories:
- Institut des Matériaux, de Microélectronique et des Nanosciences de Provence (IM2NP, Aix-Marseille Université/CNRS)
- Centre d’Elaboration de Matériaux et d’Etudes Structurales (CEMES, Université de Toulouse/CNRS)
- Laboratoire d’Hétérochimie Fondamentale et Appliquée (LHFA, Université de Toulouse/CNRS)
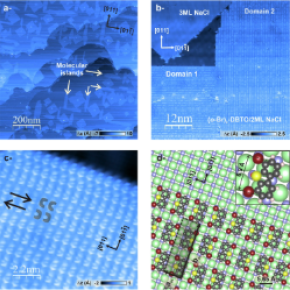
Given these limitations associated with the use of metal surfaces, a collaboration between researchers from Marseille and Toulouse has undertaken to study the transposition of a particular chemical reaction (the photoinduced deoxygenation of a dibenzothiophene S-oxide (DBTO) derivative), well known in solution, onto an insulating alkali halide surface under ultra-high vacuum and at low temperature (see figure (a)). By combining studies in solution and on the surface using scanning tunnelling microscopy (STM, see figure (b)), non-contact atomic force microscopy (nc-AFM, see figure (c)) and potential spectroscopy measurements, the researchers observed the deoxygenation of the DBTO derivative under UV irradiation at the single molecule level.

The biarylsulfoxide family from which DBTO is derived is particularly interesting for its photoreaction properties and the significant polarity of the sulfinyl S=O group. As the oxidation state of the sulphur atom varies from (0) to (-II) during this reaction, the photodesoxygenation of sulfoxides is therefore a powerful, reagent-free synthetic tool for reducing sulphur. It is conceivable that the atomic oxygen resulting from UV-induced cleavage of the S=O bond could be exploited to initiate new inter- or intramolecular oxidation reactions on the surface. These results also pave the way for photocontrolled manipulation of the charge state in purely organic compounds on surfaces. They are published in the journal Nature Communications.