What makes matter stable?
Researchers have visualised for the first time the direct effect of the Pauli exclusion principle, a fundamental feature of quantum mechanics. This principle allows ordinary matter to be stable and determines how our world is structured by endowing interactions between atoms with unique characteristics.
References :
Quantum Gas Microscopy of Fermions in the Continuum, Tim de Jongh, Joris Verstraten, Maxime Dixmerias, Cyprien Daix, Bruno Peaudecerf, Tarik Yefsah, Physical Review Letters 134, 183403 – Published 5 May, 2025.
M. Parish, A Glimpse at the Quantum Behavior of a Uniform Gas, Physics, 18, 89 (2025).
Doi : 10.1103/PhysRevLett.134.183403
Open access archive : arXiv
Electrons, protons and neutrons, which are the main building blocks of the matter around us, have one thing in common: they are fermions. These particles possess a distinctive property: it is impossible to find two of them in the same physical state. This characteristic of fermions is called the Pauli exclusion principle, named after the famous physicist Wolfgang Pauli, who explicitly formulated it exactly a century ago, earning him the Nobel Prize twenty years later. This rule, which has been observed but not proven, is fundamental to quantum mechanics and essential to understanding the world around us. It is this principle that prevents matter from collapsing, explains the properties of semiconductors and dictates the structure of the periodic table. However, as the Pauli principle concerns extraordinarily small energy and length scales, it had previously only been observed indirectly, via the effects it induces at more macroscopic scales of matter.
The present research was conducted in the following CNRS laboratories:
Laboratoire Kastler Brossel (LKB, CNRS/Collège de France/ENS-PSL/Sorbonne Université)
Laboratoire Collision Agrégats Réactivité (LCAR, CNRS/Université de Toulouse)
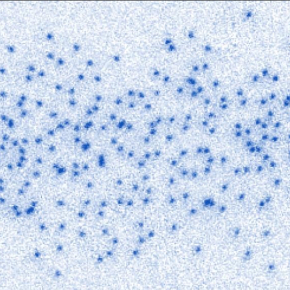
In a recently published study, scientists at the Kastler Brossel Laboratory (LKB, CNRS/Collège de France/ENS-PSL/Sorbonne Université) revealed the clearest images ever taken of the Pauli exclusion principle in action at the atomic scale. They used clouds of hundreds of 6Li atoms (which are fermions) cooled to temperatures close to absolute zero. These low temperatures make the quantum properties of the particles observable, which would otherwise be masked by their thermal agitation. The team used a technique they recently developed to image quantum waves. By first allowing the atoms to evolve freely in space, then immobilising them in an optical lattice — regularly spaced microscopic ‘cages’ created by laser light — the scientists were able to directly photograph the fermionic system, recording the position of each atom. This procedure directly revealed the Pauli exclusion principle ‘in action,’ as statistical analyses of the configurations show that the atoms avoid each other and give a statistical signature in perfect accordance with what quantum theory predicts.
In the system they studied, Pauli exclusion was the only possible interaction between atoms, revealing its effects in the purest imaginable manner. The team examined the correlations between the positions of two and three particles, revealing a significant decrease in the number of pairs and triplets of atoms located at short distances from each other, a property called the ‘Pauli hole’ and considered an unambiguous signature of the principle of the same name.
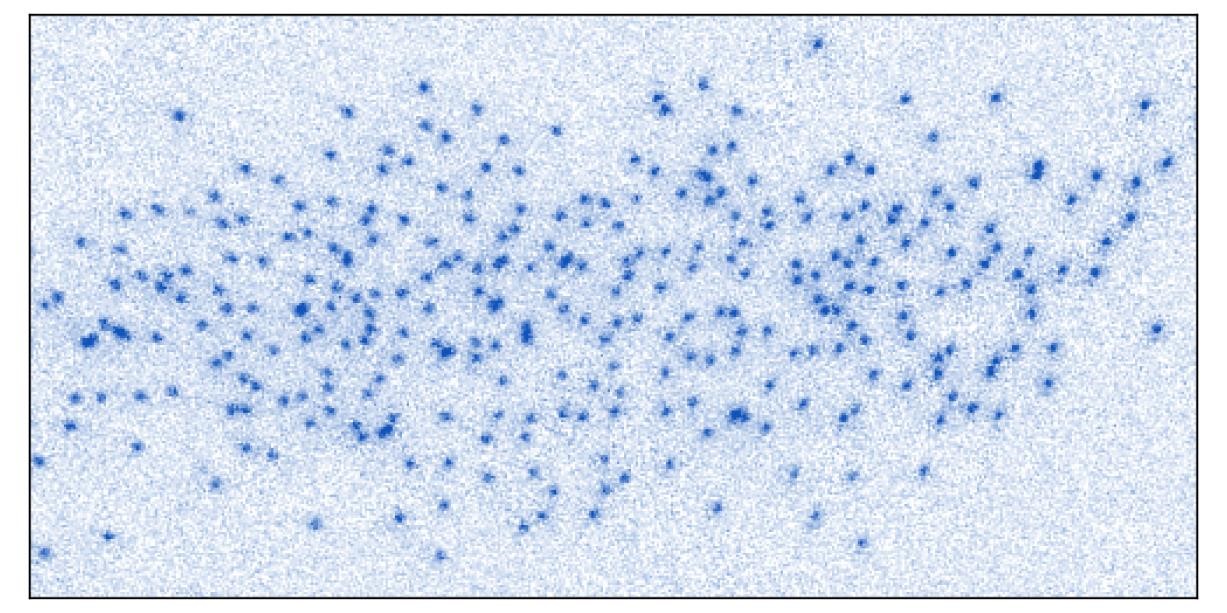
Thanks to their technique, researchers can now study much more complicated systems, such as assemblies of strongly interacting fermions, where the Pauli exclusion principle competes with particle collisions. By being able to take direct images of the configurations of their gas, this method will allow them to measure the behaviour of fermionic systems so complex that even the most powerful supercomputers cannot simulate them. This work was selected as Editor's Suggestion in Physical Review Letters, where it is published on the cover. It was also reviewed in the APS journal Physics.