A new type of intramolecular coupling
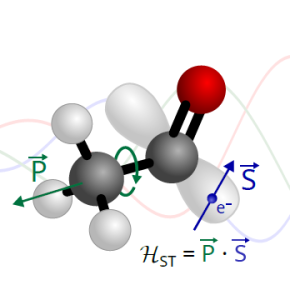
A novel interaction has been observed in the unstable radical CH₃CO, which exhibits internal rotational motion. This interaction manifests as an unprecedented coupling between the electron spin of the unpaired electron and the torsional motion of the methyl group.
References :
Evidence for Electron Spin-Torsion Coupling in the Rotational Spectrum of the CH3CO Radical, Laurent H. Coudert, Olivier Pirali, Marie-Aline Martin-Drumel, and Rosemonde Chahbazian, Luyao Zou, Roman A. Motiyenko, Laurent Margulès, Physical Review Letters, 134, 173001 – Published on 2 May 2025.
DOI : 10.1103/PhysRevLett.134.173001
Open access archive : arxiv
Molecular energy levels can be altered by various internal interactions, often subtle. In the case of the CH₃CO radical, a team of researchers from CNRS, Université Paris-Saclay, and Université de Lille has uncovered a type of coupling never before observed: between the spin of an unpaired electron and the internal rotation of a methyl group (CH₃). This so-called spin-torsion coupling significantly alters the arrangement of lines in the molecule's rotational spectrum, introducing small, characteristic shifts linked to this unique interaction.
The present research was conducted in the following CNRS laboratories:
- Institut des Sciences Moléculaires d'Orsay (ISMO, CNRS / Université Paris-Saclay)
- Laboratoire Physique des lasers, atomes et molécules (PhLAM, CNRS / Université de Lille)
CH₃CO is an "open-shell" molecule, meaning it possesses a single unpaired electron. Such species typically exhibit split spectral lines due to the interaction between the electron spin and the overall molecular rotation. However, in this case, the influence of the methyl group's torsional motion on the electron spin has also been demonstrated. To characterize this interaction, the researchers performed high-resolution analysis of the CH₃CO rotational spectrum, recorded in the millimeter and submillimeter ranges using recently developed experimental techniques.
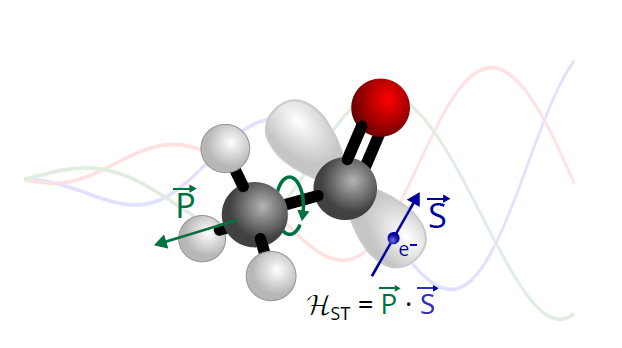
The identification of 275 rotational transitions revealed line splittings that could not be explained by conventional theoretical models. By refining their approach, the scientists developed a new model incorporating this novel type of interaction, which allowed them to reproduce the experimental data with high accuracy. The spin-torsion coupling constants obtained through fitting were found to be consistent with theoretical predictions based on the molecule’s electronic structure and known spin-rotation coupling effects, thus supporting the proposed interpretation.
This discovery paves the way for studying other non-rigid radical molecules that exhibit large-amplitude internal motions. It will also help improve spectroscopic databases used in astrochemistry for the detection of such species with radio telescopes. The CH₃CO radical itself may soon be identified in the interstellar medium, complementing its already detected cation, CH₃CO⁺.