Encapsulating drugs to reduce their cardiotoxicity
An international team has developed a new nano-platform based on self-assembled dendrimers for drug delivery with the aim of limiting hERG channel-related cardiotoxicity and improving treatment efficacy.
References :
Self-assembling dendrimer nanodrug formulations for decreased hERG-related toxicity and enhanced therapeutic efficacy, Xi Liu, Dinesh Dhumal, Patricia Santofimia-Castaño, Juan Liu, Marion Casanova, Alicia Comino Garcia-Muñoz, Teodora-Adriana Perles-Barbacaru, Abdechakour Elkihel, Wenzheng Zhang, Tom Roussel, Christina Galanakou, Jing Wu, Eleni Zerva, Nelson Dusetti, Yi Xia, Xing-Jie Liang, Angèle Viola, Juan L. Iovanna, Ling Peng, Science Advances 11,eadu9948 - Publié le 25 juin 2025.
DOI : 10.1126/sciadv.adu9948
Cardiotoxicity is a major cause of drug failure, particularly when linked to the hERG channel. It has led to the withdrawal of several drugs from the market and the failure of many molecules during the development phase. Reducing the binding of therapeutic compounds to the hERG channel is therefore a crucial issue in drug development. Scientists extensively explored nanotechnologies for drug delivery in order to reduce systemic toxicity and improve therapeutic efficacy. However, few studies on these strategies specifically target hERG-related cardiotoxicity.
An international team involving three CNRS laboratories has designed and developed a nano-platform capable of encapsulating drugs to limit their cardiotoxicity. This platform previously studied for producing contrast agents for biomedical imaging or fluorinated nanovectors for simultaneously imaging and treating cancerous tumors, is based on amphiphilic dendrimers. Dendrimers are molecules with a very precise structure that branches out from a central core to its periphery, like a tree. The platform exploits their precise molecular architecture, multivalent cooperativity, and self-assembly behavior. The dendrimers, which self-assemble into nanomicelles (spherical aggregates of amphiphilic molecules), have adaptable properties, a uniform size, and a high drug loading capacity. Scientists can encapsulate molecules at the core of these nanosystems for effective delivery while limiting their binding to the hERG channel.
The present study was carried out in the following CNRS laboratories:
- Centre Interdisciplinaire de Nanoscience de Marseille (CINaM, CNRS/Aix-Marseille Université)
- Centre de Recherche en Cancérologie de Marseille, (CRCM, CNRS/Institut Paoli-Calmettes/Aix-Marseille Université)
- Centre de Résonance Magnétique Biologique et Médicale (CRMBM, Aix Marseille University/CNRS)
Researchers tested three molecules known for their binding hERG binding to validate this strategy: chloroquine (CQ), used in the treatment of malaria; doxorubicin (DOX), an anti-cancer drug; and finally, a nuclear protein 1 inhibitor, ZZW115, an emerging molecule with anti-cancer properties. Self-assembled dendrimers enabled the effective encapsulation of these three molecules, despite their very different structures and properties, confirming their broad applicability and versatility for drug delivery. Remarkably, these nanoformulations significantly reduced the binding affinity of the three molecules to the hERG channel. These results indicate a clear reduction in potential cardiac risk.
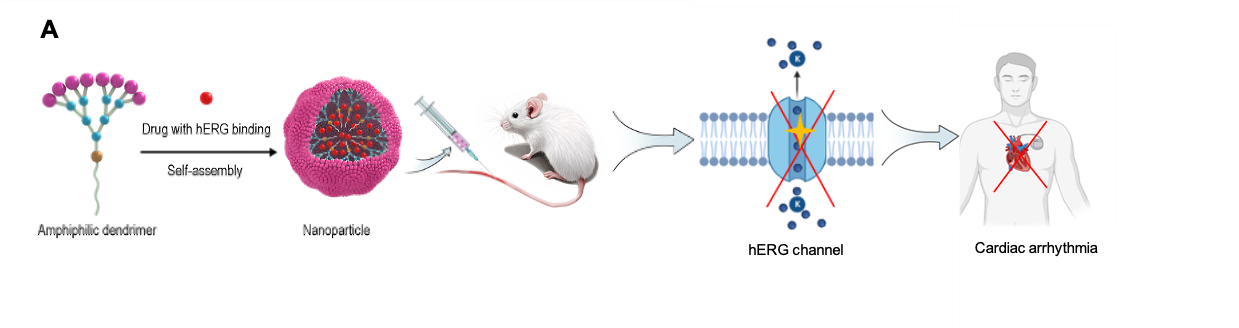
In addition to reduced cardiotoxicity, these nanodrugs have demonstrated highly favorable pharmaceutical properties, such as small, uniform particle size and prolonged circulation time in the bloodstream, enabling effective targeting of disease sites in the body. In vivo imaging confirmed increased accumulation of antimalarial treatment in the liver and of anticancer drugs in tumors. Animal studies confirmed reduced toxicity and improved efficacy, including increased survival in the malaria model and reduced tumor growth in pancreatic cancer models.
This work represents an important step forward to improve drug delivery. It demonstrats that self-assembled dendrimer-based nanoplatforms can effectively reduce hERG-related cardiotoxicity while enhancing therapeutic effects in various drug classes and disease models. Not only do these nanoplatforms act as effective delivery systems, but they also function as molecular shields that minimize cardiac risk without the need to chemically modify drug candidates. This innovative approach has the potential to revive promising compounds whose development had been halted due to cardiotoxicity issues and accelerate their transfer to the clinic.
